Notes-(01)
4. sp2 Hybridization
4.2. Shape and Bond Angle
o Electrons repel one another
o \(sp^2\) hybrid orbitals are placed as far apart as possible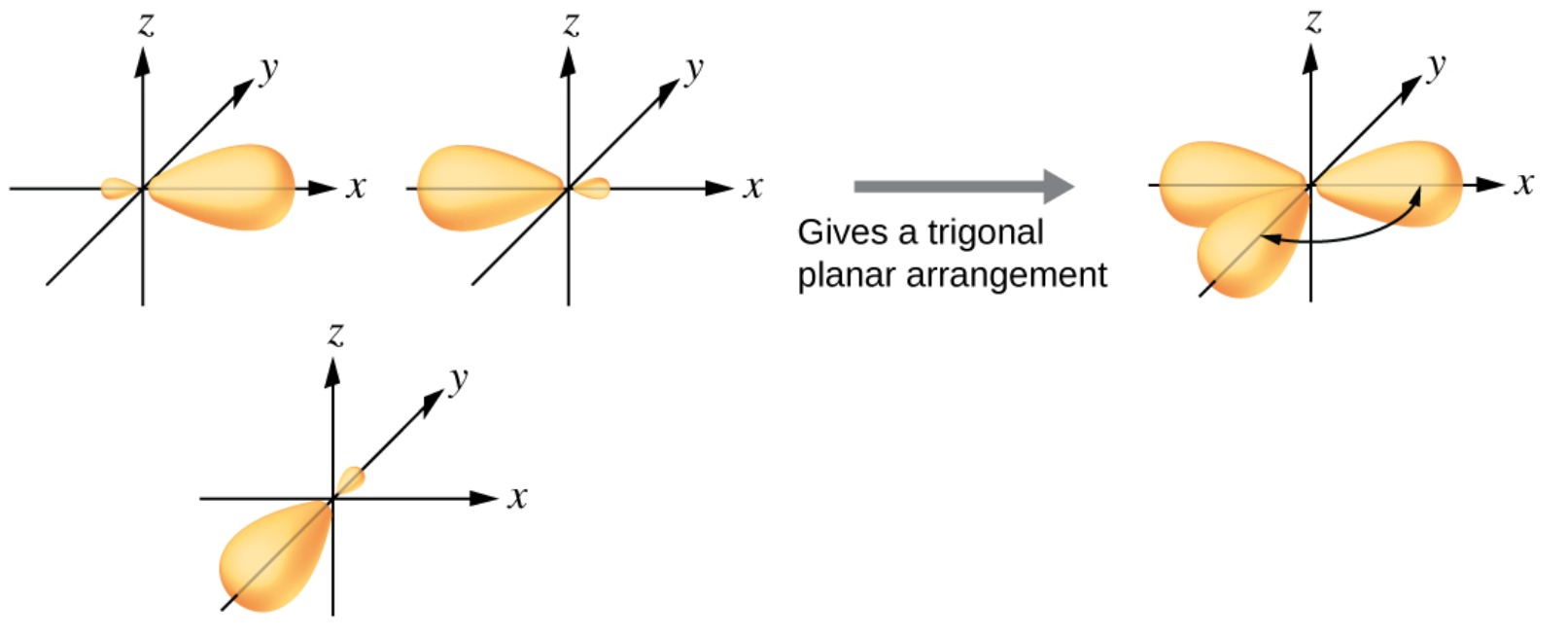
Shape: Trigonar planar
Bond Angle (Around C): 120°
o Electrons repel one another
o \(sp^2\) hybrid orbitals are placed as far apart as possible
Shape: Trigonar planar
Bond Angle (Around C): 120°