Constituitional Isomerism
| Site: | Prefrontal Learning Center |
| Course: | (a) describe constitutional (structural) isomerism |
| Book: | Constituitional Isomerism |
| Printed by: | Guest user |
| Date: | Sunday, 24 November 2024, 6:00 PM |
1. Constitutional (Structural) Isomerism
Isomers have
o Same molecular formula
01 Multiple Choice
2. Chain Isomerism
Isomers have
o Different structural formula
2.1. C4H10O (Consider ether only)
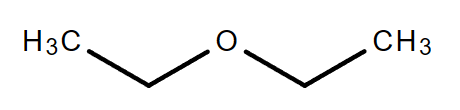 |
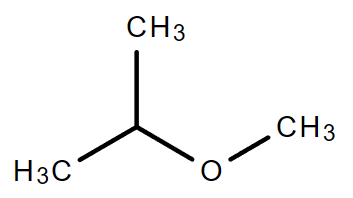 |
|
| Ethoxyethane | 1-Methoxypropane | 2-Methoxypropane |
2.2. C5H12
Molecular formula: C5H12
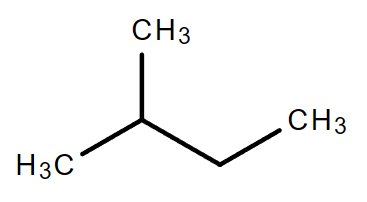 |
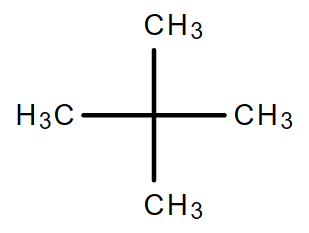 |
|
| Pentane | Isopentane | Neopentane |
2.3. C6H14
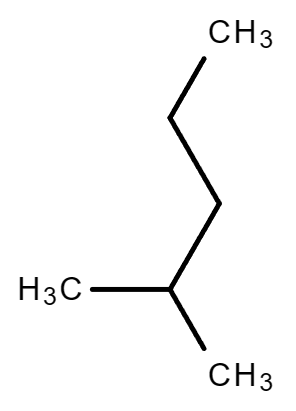 |
||
| Hexane | 2-Methylpentane | 3-Methylpentane |
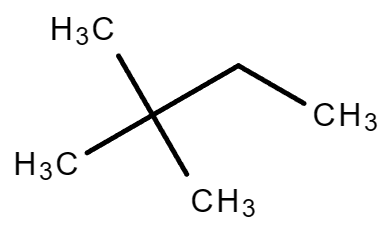 |
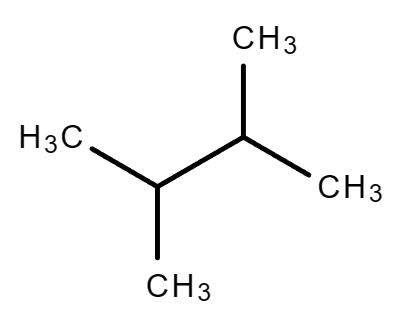 |
|
| 2,2-Dimethylbutane | 2,3-Dimethylbutane |
3. Position Isomerism
o Same functional group that differ in its position
3.1. C3H8O (Consider alcohol and non-cyclical compound only)
Molecular formula: C3H8O
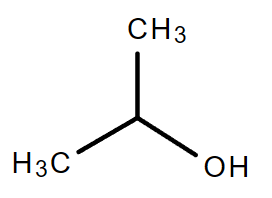 |
|
| 1-Propanol | 2-Propanol |
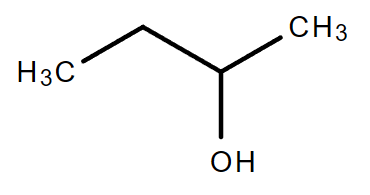
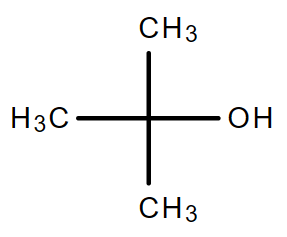
3.2. C4H10O (Consider alcohol and non-cyclical compound only)
| Chain isomerism | Position isomerism | Position isomerism |
 |
 |
|
| 1-Butanol | 2-Butanol | 2-Methyl-2-propanol |
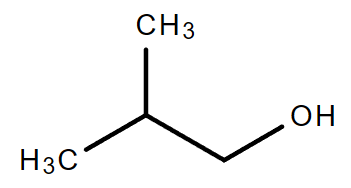 |
||
| 2-Methyl-1-propanol |
3.3. C5H10 (Consider alkene and non-cyclical compound only)
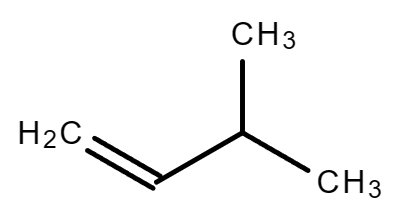 |
||
| 1-Pentene | 2-Methyl-1-butene | 3-Methyl-1-butene |
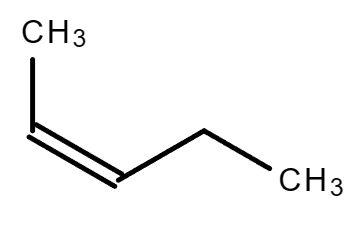 cis cis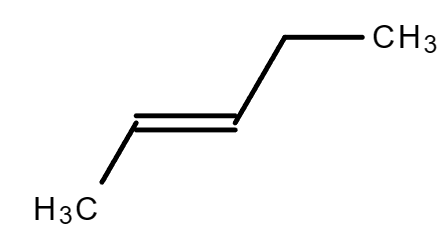 trans trans |
 |
|
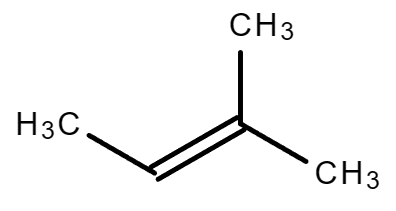
| 2-Pentene | 2-Methyl-2-butene |
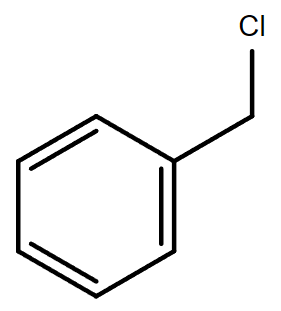
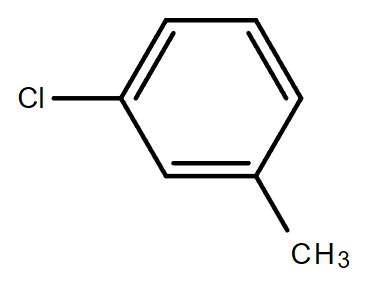
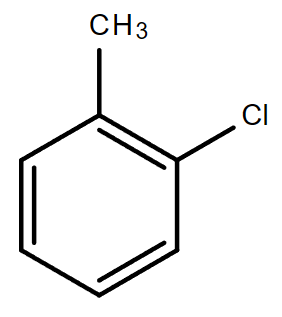
3.4. C7H7Cl (Contains a benzene ring)
Molecular formula: C7H7Cl
 |
 |
 |
|
|
(Chloromethyl) |
1-Chloro-3-methylben |
1-Chloro-4-methylben |
1-Chloro-2-methylben |
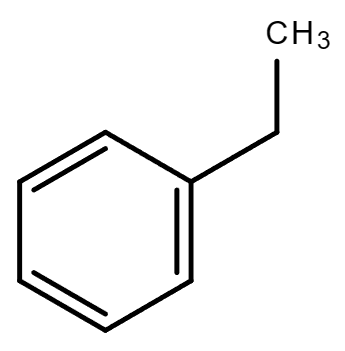
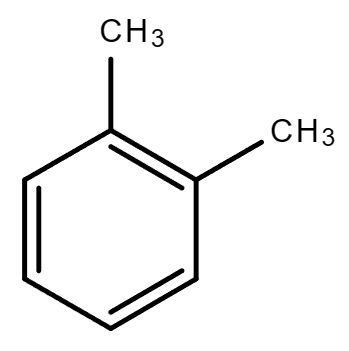
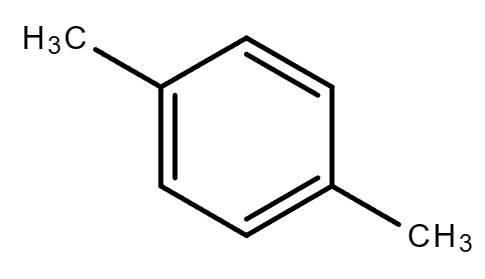
3.5. C8H10 (Contains a benzene ring)
 |
 |
 |
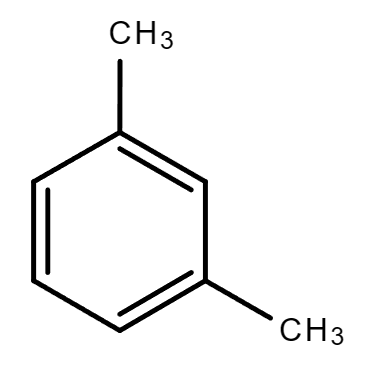 |
| Ethylbenzene | 1,2-Dimethylbenzene (o-Xylene) | 1,4-Dimethylbenzene (p-Xylene) | 1,3-Dimethylbenzene (m-Xylene) |
4. Functional Group Isomerism
Isomers have
o Different functional group
4.1. C2H6O
Molecular formula: C2H6O
| Ethanol | Methoxymethane |
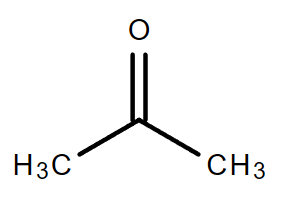
4.2. C3H6O
Molecular formula: C3H6O
 |
|
| Acetone | Propionaldehyde |
4.3. C3H6O2
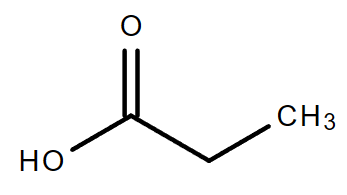 |
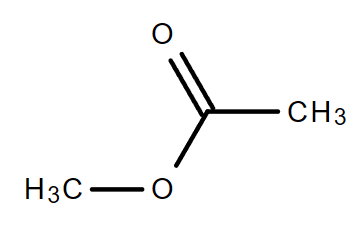 |
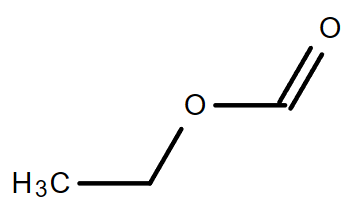 |
| Propanoic acid | Methyl ethanoate | Ethyl methanoate |