Notes
| Site: | Prefrontal Learning Center |
| Course: | 03 Mechanism |
| Book: | Notes |
| Printed by: | Guest user |
| Date: | Friday, 20 September 2024, 5:33 AM |
1. Clock Reaction (Initial rate method)
1.1. Measure Reaction
Objective:
To determine the average rate of the reaction by measuring the time taken for the reaction mixture to produce __________ so that a mark on a piece of white paper becomes obscured.
1.2. Set-up
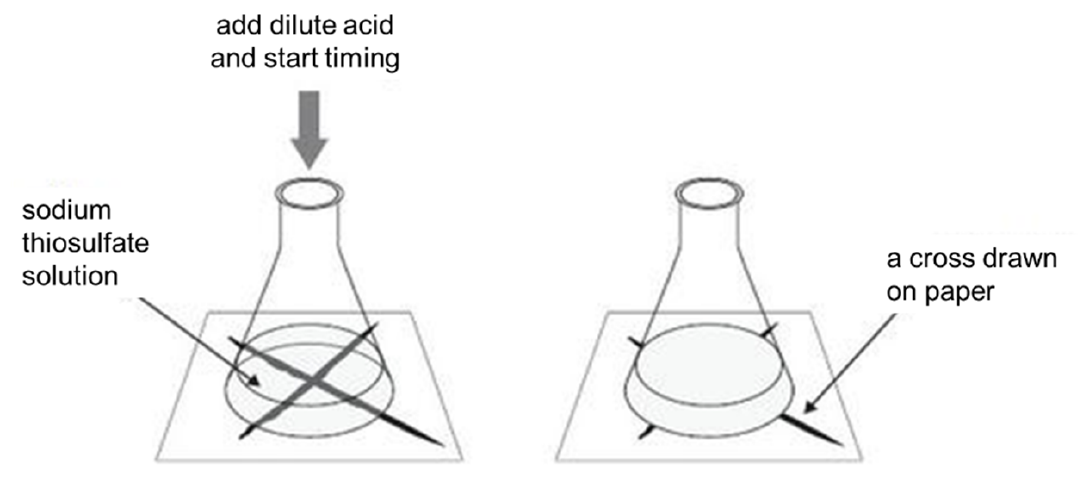
1.3. When to start the stopwatch
o Start the stopwatch when the reactants are mixed
1.4. When to stop the stopwatch
o Stop the stopwatch when the "X" is just obscured from view
1.5. Procedure to determine the order of reaction with respect to S2O3(2-) ions
| Experiment | \([H^+]\) / mol dm\(^{-3}\) | \(\text{S}_2\text{O}_3^{2-}\) / mol dm\(^{-3}\) | Time (s) |
| 1 | 0.001 | 0.001 | 120 |
| 2 | 0.001 | 0.002 | 60 |
Calculate Rate of Reaction
| Experiment | \([H^+]\) / mol dm\(^{-3}\) | \(\text{S}_2\text{O}_3^{2-}\) / mol dm\(^{-3}\) | Time (s) | Rate of Reaction (1/time) |
| 1 | 0.001 | 0.001 | 120 | \( \frac{1}{120} \approx 0.0083 \) s\(^{-1}\) |
| 2 | 0.001 | 0.002 | 60 | \( \frac{1}{60} \approx 0.0167 \) s\(^{-1}\) |
W.r.t S2O32− and H+
Compare Expt 1 and Expt 2 where [H+] is the same.
When \([\text{S}_2\text{O}_3^{2-}]_{\text{Expt 2}}\) is twice of
\([\text{S}_2\text{O}_3^{2-}]_{\text{Expt 1}}\)
o Rate (2) is twice of rate (1)
o Order w.r.t \(\text{S}_2\text{O}_3^{2-}\) is one.
1.6. Iodine Clock Reaction: Hydrogen Peroxide and Iodide Ion with Fixed Sodium Thiosulfate
Chemical equation
o \( \text{H}_2\text{O}_2 + 2 \text{I}^- + 2 \text{H}^+ \rightarrow \text{I}_2 + 2 \text{H}_2\text{O} \)
o \(\text{I}_2 + 2 \text{S}_2\text{O}_3^{2-} \rightarrow 2 \text{I}^- + \text{S}_4\text{O}_6^{2-} \)
o Indicator: Starch
Color change
o Initial solution
- Iodine (I2\text{I}_2) is produced and immediately reacts with thiosulfate ions \(\text{S}_2\text{O}_3^{2-}\)
- Colorless
o Final solution
- Thiosulfate ions are entirely consumed and free iodine accumulates in solution
- Dark blue