01 Empirical, molecular and structural formulae
Candidates are expected to be able to interpret and use the following types of representations in the description of organic molecules.
The examples given are for the compound (+)-lactic acid.
Empirical Formula: simplest ratio of the number of atoms of the elements present in one molecule, e.g. CH2O
Molecular Formula: actual number of atoms of the elements present in one molecule, e.g. C3H6O3
Structural Formula: shows how the constituent atoms of a molecule are joined together with minimal detail, using conventional groups, for an unambiguous structure, e.g. CH3CH(OH)CO2H
Full Structural or Displayed Formula: detailed structure of molecule showing the relative placing of atoms and the number of bonds between them, e.g.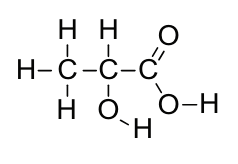
Skeletal Formula: simplified representation of an organic formula derived from the structural formula by removing hydrogen atoms (and their associated bonds) and carbon atoms from alkyl chains, leaving just the carbon-carbon bonds in the carbon skeleton and the associated functional groups
Skeletal or partial skeletal representations may be used in question papers and are acceptable in candidates’ answers where they are unambiguous, e.g.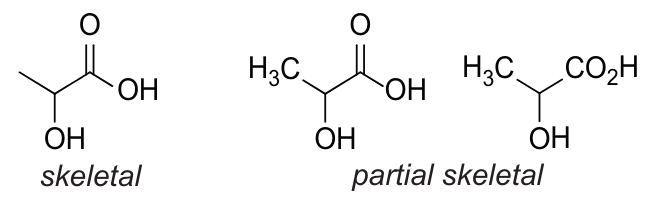
The convention 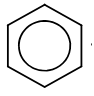 for representing the aromatic ring is preferred.
for representing the aromatic ring is preferred.
Stereochemical Formula: show spatial arrangement of bonds, atoms and groups in molecule in 3-D, e.g.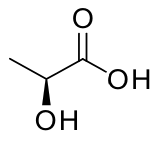
When drawing a pair of optical isomers, candidates should indicate the three-dimensional structures according to the convention used in the example below.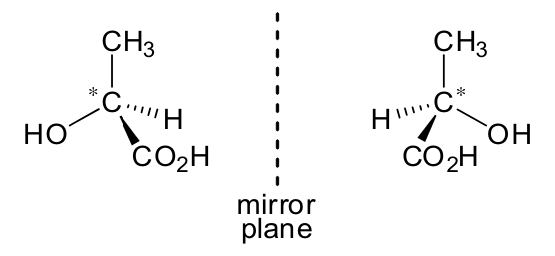
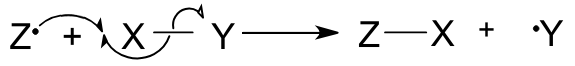
Candidates are expected to be able to interpret and use the curly arrow notation to represent the movement of electrons in organic reaction mechanisms.
For movement of a pair of electrons (full arrow) :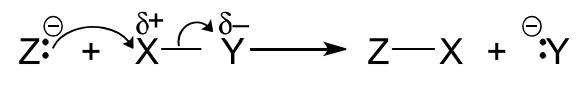
For movement of a single unpaired electron (half arrow) :