Notes-(01)
Completion requirements
(d) describe sp3 hybridisation, as in ethane molecule, sp2 hybridisation, as in ethene and benzene molecules, and sp hybridisation, as in ethyne molecule
(e) explain the shapes of, and bond angles in, the ethane, ethene, benzene, and ethyne molecules in relation to σ and π carbon-carbon bonds
5. sp Hybridisation
5.2. Shape and Bond Angle
o Electrons repel one another thus the \(sp\) hybrid orbitals are placed as far apart as possible by adopting the linear arrangement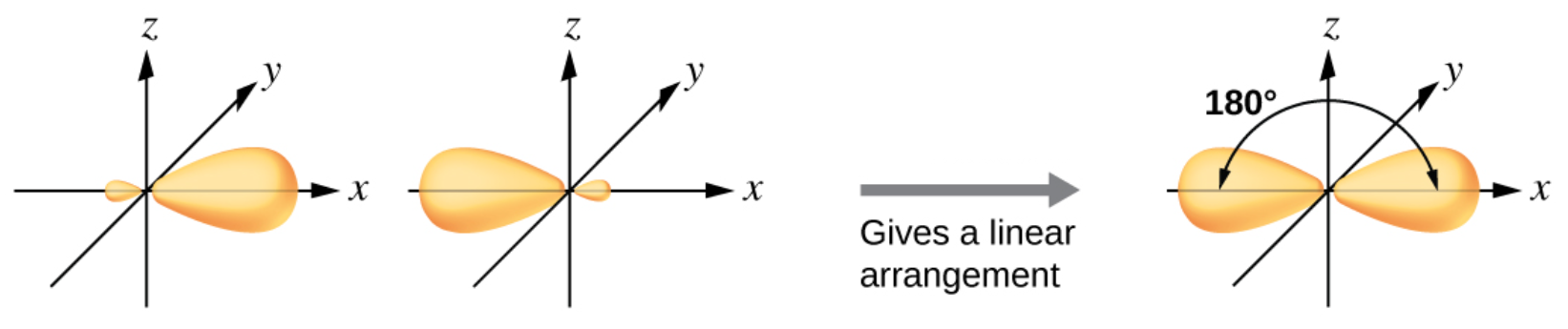
Shape: Linear
Bond Angle (Around C): 180°