Cis-trans Isomerism
Completion requirements
2. Identifying Cis or Trans Isomers
Consider a compound with 4 different groups attached to the C = C bond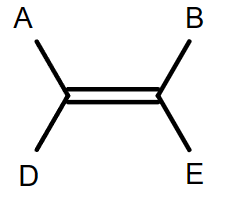
- Consider each of the double bonds separately.
- Identify the two atoms directly attached to each carbon in the double bond
- If the two atoms with larger atomic numbers are on the same side:
- It is a cis isomer.
- If the two atoms with larger atomic numbers are on opposite sides:
- It is a trans isomer.
Note: Show clear trigonal planar shape around the 2C atoms of C=C bond
01 Multiple Choice